Hepatocellular carcinoma is a common disease
Cancer is the second most common cause of death worldwide and liver cancer is the second most common cause of cancer death. It accounted for 788,000 deaths in 2015 according to the World Health Organization: http://www.who.int/news-room/fact-sheets/detail/cancer. Hepatocellular carcinoma (HCC) is the most common type of liver cancer accounting for approximately 90% of all liver cancers, with average survival rates between 6 and 20 months.
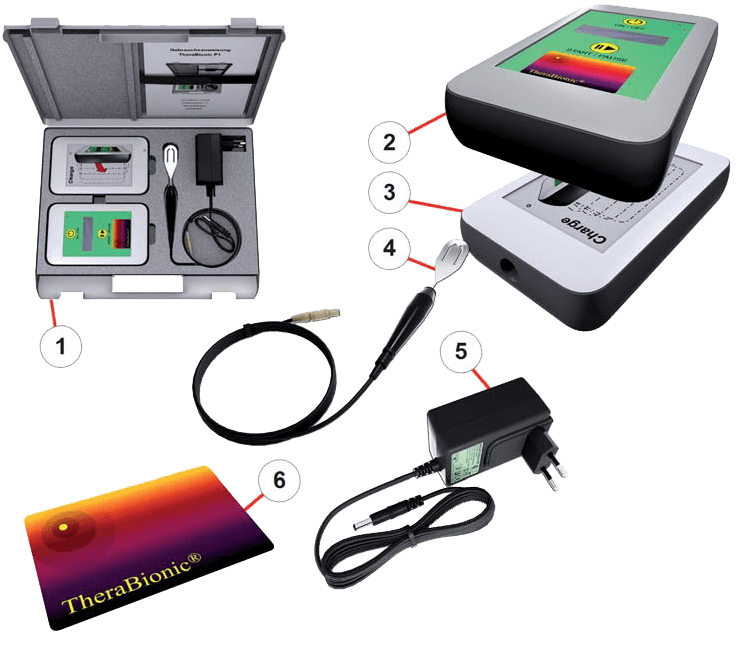
Treatment with the TheraBionic P1
Until recently, only symptomatic treatment was advised for patients who have failed or are intolerant to first line and second line therapies for advanced hepatocellular carcinoma.
The TheraBionic P1 device enables a new and innovative treatment approach. It is used for the systemic treatment of patients with advanced hepatocellular carcinoma who have either failed first and second line therapies or are intolerant of first and second line therapies.
Comparison with the results from randomized studies conducted in patients with advanced hepatocellular carcinoma
This report describes a methodologically sound assessment of the therapeutic efficacy of the TheraBionic (TB) device by way of a statistical analysis of several studies. In this report the results of a single arm TheraBionic study are compared with control groups and treatment groups from two other studies. We examine three outcomes:
- Progression Free Survival
- Response Rate
- Overall Survival Among Patients who are not Child Pugh Class B

